Introduction
Thermodynamics has long been a cornerstone of classical physics, providing essential principles for understanding energy transfer and transformation. From steam engines to refrigerators, thermodynamics governs the behavior of macroscopic systems. However, as we delve deeper into the quantum realm, where particles are mere whispers of existence, the conventional rules of thermodynamics start to blur and bend.
In this blog post, we’ll embark on a journey into the exciting world of quantum thermodynamics. We’ll explore how energy and entropy behave at the nanoscale, where quantum effects reign supreme. To kick things off, let’s revisit the basics of thermodynamics and then delve into the peculiarities of quantum physics.
Thermodynamics: A Brief Recap
Before we dive into the quantum realm, it’s essential to have a firm grasp of classical thermodynamics. The discipline is built upon four fundamental laws:
The Zeroth Law: If two systems are in thermal equilibrium with a third system, they are in equilibrium with each other.
The First Law (Conservation of Energy): Energy cannot be created or destroyed; it can only change forms. This law gave rise to the concept of internal energy and the famous equation, ΔU = Q – W, where ΔU is the change in internal energy, Q is heat added to the system, and W is work done by the system.
The Second Law (Entropy): The total entropy (disorder) of an isolated system can never decrease over time; it can only increase or remain constant. This law introduced the concept of entropy, denoted by S, which characterizes the dispersal of energy in a system.
The Third Law (Absolute Zero): As the temperature of a system approaches absolute zero (0 Kelvin), the entropy of the system approaches a minimum constant value.
These laws form the foundation of classical thermodynamics, governing our understanding of heat engines, refrigerators, and countless other everyday processes. However, when we transition to the quantum realm, things get intriguingly complex.
Quantum Mechanics: A Primer
Quantum mechanics, often dubbed quantum physics, is the branch of physics that deals with the behavior of particles at the smallest scales. It’s a realm where classical intuitions break down, and particles can exist in superposition states, tunnel through barriers, and exhibit entanglement. Let’s briefly touch upon some key quantum concepts that will be relevant to our discussion of quantum thermodynamics.
Superposition: In quantum mechanics, particles can exist in multiple states simultaneously, known as superposition. For example, an electron can be in a superposition of spin-up and spin-down states.
Quantum Entanglement: When two or more particles become entangled, their properties become interconnected. Changes in one particle’s state instantaneously affect the state of the other, regardless of the distance separating them.
Quantum Tunneling: Particles can penetrate energy barriers that classical physics would deem insurmountable. This phenomenon, known as quantum tunneling, plays a crucial role in various quantum devices.
Quantum Uncertainty: Heisenberg’s Uncertainty Principle states that there is a fundamental limit to how precisely we can simultaneously measure a particle’s position and momentum. The more accurately we know one property, the less accurately we can know the other.
These quantum concepts challenge our classical intuitions and set the stage for the intriguing world of quantum thermodynamics.
Quantum Thermodynamics: Where Energy Meets Entropy
Now that we have a basic understanding of classical thermodynamics and quantum mechanics, we can explore how these two realms intersect in quantum thermodynamics. At the nanoscale, where particles obey quantum laws, energy and entropy take on new forms and behaviors.
Quantum Energy Levels: In classical thermodynamics, energy levels are continuous, allowing for a smooth distribution of energy. However, in the quantum world, energy levels are quantized, meaning they come in discrete, quantifiable units. This quantization leads to the concept of energy “packets” or quanta, which underlie many quantum phenomena.
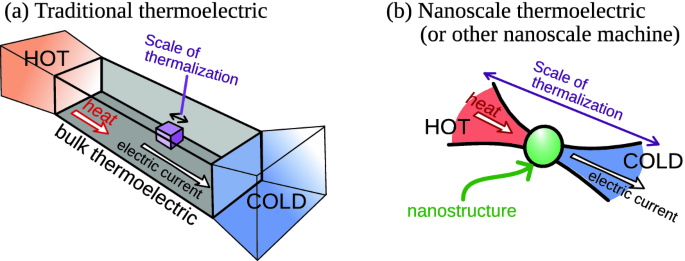
Quantum Heat Baths: Classical thermodynamics often assumes the presence of an infinite heat bath, a reservoir with an inexhaustible supply of energy. In the quantum realm, such assumptions no longer hold. Heat baths become finite, and their energy fluctuations become significant, leading to phenomena like temperature fluctuations and the breakdown of thermodynamic equilibrium.
Quantum Engines: In classical thermodynamics, heat engines operate based on the principles of the Carnot cycle, which relies on the transfer of heat between two temperature reservoirs. Quantum engines, on the other hand, exploit quantum effects such as tunneling and entanglement to perform work more efficiently and in ways that classical engines cannot.
Quantum Maxwell’s Demon: Maxwell’s demon is a famous thought experiment in classical thermodynamics, where an imaginary demon could sort fast-moving particles from slow-moving ones to violate the second law of thermodynamics. In the quantum realm, the demon’s actions can be performed with greater precision and reveal the subtleties of information thermodynamics.
Quantum Entropy: Quantum entanglement introduces a new dimension to entropy. Entangled particles share a quantum correlation that classical systems lack, and this correlation affects the entropy of the entire system. Understanding and manipulating quantum entropy are critical in quantum information theory and quantum computing.
Quantum Refrigeration: The principles of quantum thermodynamics also apply to quantum refrigeration, where tiny quantum refrigerators can cool down systems to ultralow temperatures, approaching absolute zero. This has implications for both fundamental physics and practical applications in fields like quantum computing.
The Challenges of Quantum Thermodynamics
While quantum thermodynamics offers exciting possibilities, it also presents formidable challenges. For instance, reconciling the seemingly contradictory principles of quantum mechanics and thermodynamics has been a topic of intense research and debate. One of the central challenges is the formulation of quantum analogs of the classical laws of thermodynamics.
Researchers are working on developing a consistent framework for quantum thermodynamics that can encompass the unique features of quantum systems while still preserving the core principles of energy conservation and entropy increase. This endeavor has led to the emergence of quantum thermodynamic formalisms, such as quantum thermodynamic cycles and fluctuation theorems, which aim to bridge the gap between quantum mechanics and thermodynamics.
Additionally, the practical implementation of quantum thermodynamic processes poses significant engineering and technological hurdles. Quantum devices, such as quantum heat engines and refrigerators, are in their infancy, and researchers are striving to harness the full potential of quantum effects for real-world applications.
Conclusion
Quantum thermodynamics is a captivating and rapidly evolving field that explores the intricate relationship between energy and entropy at the nanoscale. It challenges our classical understanding of thermodynamics by introducing the bizarre and fascinating phenomena of quantum physics.
As researchers continue to push the boundaries of quantum thermodynamics, we can anticipate breakthroughs with far-reaching implications. These may include the development of more efficient energy conversion technologies, the advancement of quantum computing, and a deeper understanding of the fundamental laws governing our universe.
In this blog post, we’ve only scratched the surface of the rich and complex world of quantum thermodynamics. It is a field ripe with possibilities, where quantum mysteries meet the timeless laws of thermodynamics, promising new insights into the fundamental workings of the universe.