Introduction
Quantum physics, with its peculiar and often perplexing phenomena, has always been a subject of intrigue and fascination. Among the many mind-bending principles that govern the quantum world, Heisenberg’s Uncertainty Principle takes center stage. Coined by the German physicist Werner Heisenberg in 1927, this principle has fundamentally altered our understanding of nature at the smallest scales.
At its core, the Uncertainty Principle challenges the classical notion that we can precisely determine both the position and momentum of a particle simultaneously. Instead, it reveals an inherent limitation, suggesting that there is a fundamental uncertainty in these measurements. To truly appreciate the significance of this principle, we must embark on a journey through the historical development of quantum mechanics and understand the delicate dance between particles and waves.
The Birth of Quantum Mechanics
To comprehend the Uncertainty Principle, we must first grasp the context in which it emerged. The early 20th century was a revolutionary period in physics. Classical physics, which had successfully explained the behavior of objects on macroscopic scales, was hitting its limits when it came to the subatomic world.
Quantum mechanics emerged as a groundbreaking theory, pioneered by luminaries like Max Planck, Albert Einstein, Niels Bohr, and, of course, Werner Heisenberg. It introduced a paradigm shift in our understanding of the behavior of particles and waves, challenging the determinism of classical physics and ushering in a new era of probabilistic predictions.
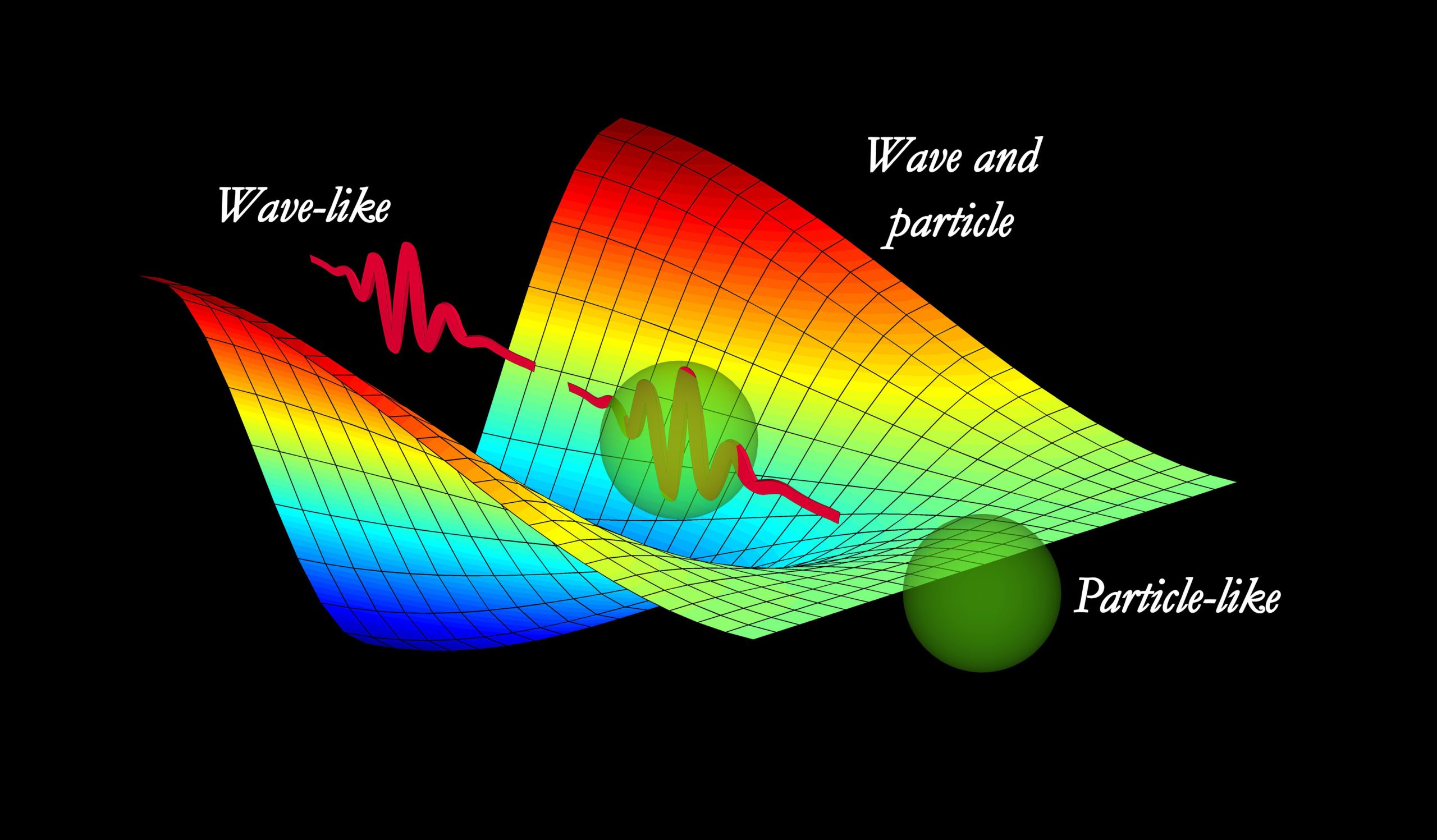
One of the key precursors to the Uncertainty Principle was the wave-particle duality, as proposed by Louis de Broglie in 1924. This concept suggested that particles, such as electrons, exhibit both wave-like and particle-like properties. It was a revolutionary idea that laid the foundation for Heisenberg’s subsequent work.
Heisenberg’s Breakthrough
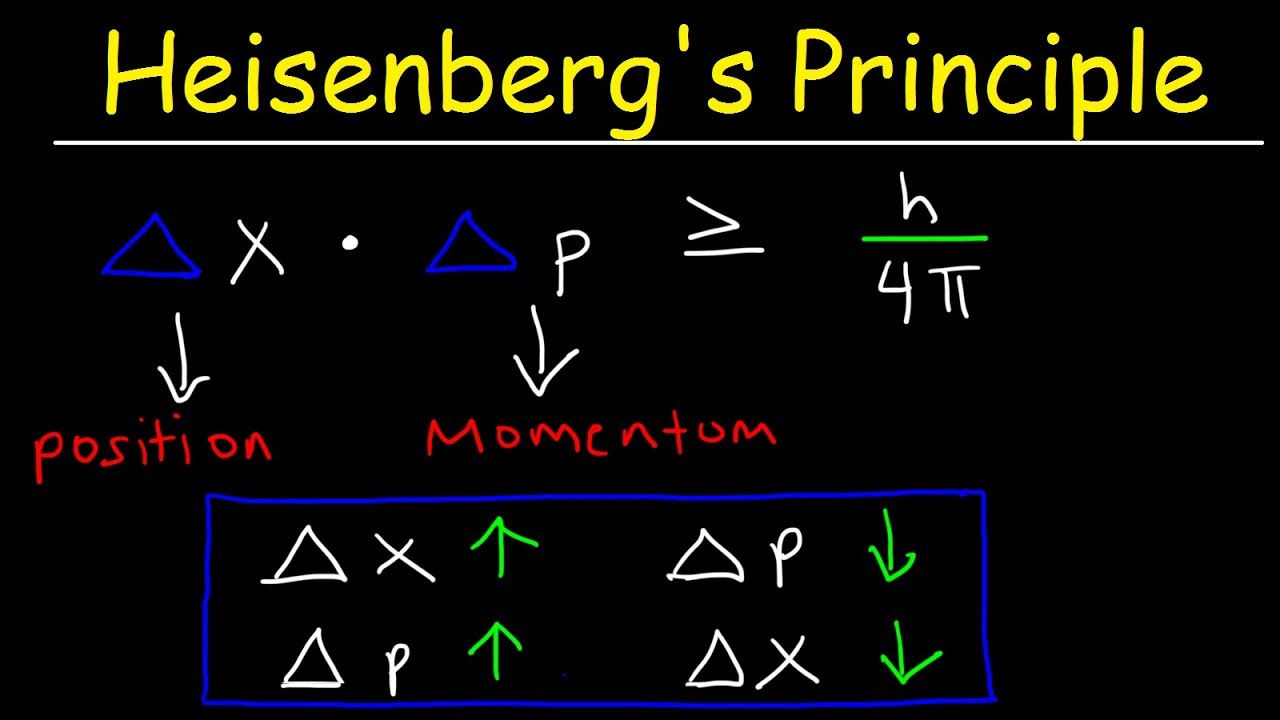
In 1927, Werner Heisenberg introduced the world to his Uncertainty Principle in a groundbreaking paper titled “Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik” or “On the Perceptual Content of Quantum Theoretical Kinematics and Mechanics.” The essence of this principle can be distilled into a simple statement: It is impossible to precisely know both the position (x) and momentum (p) of a particle simultaneously.
Mathematically, the Uncertainty Principle can be expressed as:
Δx * Δp ≥ ħ/2
Here, Δx represents the uncertainty in position, Δp represents the uncertainty in momentum, and ħ (pronounced “h-bar”) is the reduced Planck constant. This inequality implies that the more accurately we measure a particle’s position, the less accurately we can know its momentum, and vice versa.
The Philosophical Implications
Heisenberg’s Uncertainty Principle transcends its mathematical formulation; it has profound philosophical implications that continue to intrigue scientists and philosophers alike. It challenges our classical intuition about a clockwork universe in which every parameter of a particle’s motion could, in principle, be determined with perfect precision.
One interpretation of the Uncertainty Principle is the idea of “complementarity” put forth by Niels Bohr. According to Bohr, particles exhibit different behaviors depending on how they are observed. In some experiments, particles behave more like particles, and in others, they exhibit wave-like behavior. This duality is not a limitation of our measuring instruments but an intrinsic property of the quantum world.
Experimental Evidence
The Uncertainty Principle may sound abstract and theoretical, but it has been confirmed through numerous experiments. One of the most famous experiments demonstrating this principle is the double-slit experiment.
In the double-slit experiment, particles such as electrons are fired at a barrier with two slits. Behind the barrier, a screen captures the pattern of particles that pass through the slits and strike it. Remarkably, when particles are sent through one slit at a time, they create a pattern on the screen that resembles the interference pattern of waves. This suggests that particles have a wave-like nature.
However, when both slits are open simultaneously, something astonishing occurs. The particles behave as if they are passing through both slits at once, creating an interference pattern. This implies that, in this configuration, particles exhibit a wave-like behavior. But when we try to determine which slit a particle goes through, the interference pattern disappears, and the particles behave more like individual particles.
This experiment vividly illustrates the complementarity and inherent uncertainty associated with particles in the quantum realm.
Implications Beyond Physics
While Heisenberg’s Uncertainty Principle has profound implications for our understanding of the quantum world, its influence extends far beyond the realm of physics. It has inspired discussions in philosophy, metaphysics, and even popular culture.
In philosophy, the Uncertainty Principle has sparked debates about the nature of reality and the limitations of human knowledge. It challenges the classical notion of a deterministic universe and raises questions about free will and determinism. Philosophers have grappled with the idea that the act of observation can alter the behavior of quantum particles, blurring the line between observer and observed.
In popular culture, the Uncertainty Principle has made its mark in various forms. From references in science fiction literature to appearances in movies like “The Matrix” and “Interstellar,” it has captured the imagination of audiences worldwide. Its mystique and intrigue continue to make it a go-to concept for storytellers seeking to explore the boundaries of human understanding.
Conclusion: Embracing the Uncertainty
Heisenberg’s Uncertainty Principle remains a cornerstone of quantum physics, pushing the boundaries of our comprehension and challenging our classical intuitions. It invites us to embrace the inherent uncertainty of the quantum world and to question our assumptions about the nature of reality.
As we journey through the intricacies of quantum mechanics, we must remember that the Uncertainty Principle is not a limitation imposed by our tools or our understanding; it is a fundamental feature of the universe itself. It reminds us that, at the smallest scales, the universe dances to a different tune, one where certainty gives way to probability, and the act of observation shapes the very reality we seek to understand.
In the words of Werner Heisenberg himself, “The more precisely the position is determined, the less precisely the momentum is known in this instant, and vice versa.” Embracing this uncertainty, we continue to explore the quantum mysteries that have fascinated generations of scientists and thinkers, knowing that the journey to unravel the secrets of the universe is as boundless as the universe itself.