Introduction:
Thermodynamics is a branch of physics that deals with the transfer of energy between systems. It is a fundamental concept in science and has applications in a wide range of fields, including engineering, chemistry, and biology.
The Laws of Thermodynamics:
The study of thermodynamics is based on four laws, each of which describes a fundamental principle of energy transfer.
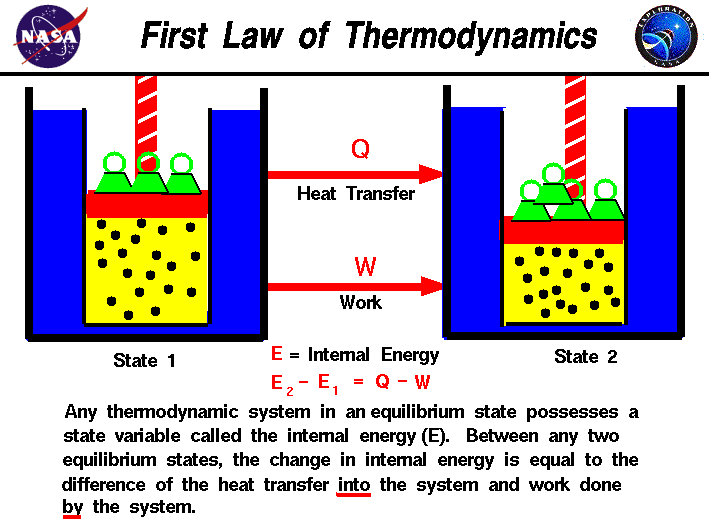
The First Law of Thermodynamics: This law states that energy cannot be created or destroyed, only transferred or converted from one form to another.
The Second Law of Thermodynamics: This law states that the total entropy of a system and its surroundings always increases over time.
The Third Law of Thermodynamics: This law states that as a system approaches absolute zero, its entropy approaches a minimum value.
The Zeroth Law of Thermodynamics: This law states that if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
Applications of Thermodynamics:
The principles of thermodynamics have many applications in everyday life, from the design of engines and refrigerators to the study of ecosystems and climate change. For example, the Second Law of Thermodynamics explains why it is impossible to build a machine that is 100% efficient, as some energy will always be lost as heat.
Thermodynamics in Chemistry:
Thermodynamics is also important in chemistry, as it provides a framework for understanding chemical reactions and the behavior of substances at different temperatures and pressures. The concept of enthalpy, which describes the heat content of a system, is fundamental to many chemical reactions.
Thermodynamics in Biology:
Finally, thermodynamics plays a crucial role in understanding biological systems, from the way cells use energy to the behavior of ecosystems. The concept of entropy, for example, can be used to explain why living organisms require a constant input of energy to maintain order and complexity.
Conclusion:
In conclusion, thermodynamics is a fundamental concept in physics that has important applications in a wide range of fields, from engineering to biology. By understanding the laws of thermodynamics and their applications, we can gain a deeper appreciation for the way energy is transferred between systems and the effects of these transfers on the physical properties of matter. Thermodynamics is a rich and complex field of study that continues to have a profound impact on our understanding of the natural world.